Background: Patients (pts) with CLL/SLL whose tumor exhibits the deletion of chromosome 17p13.1 [del(17p)] have an unfavorable prognosis and respond poorly to standard chemoimmunotherapy. Zanubrutinib (BGB-3111) is an investigational, next-generation Bruton tyrosine kinase (BTK) inhibitor. In the ASPEN study of pts with Waldenström macroglobulinemia, zanubrutinib was associated with important safety advantages compared to ibrutinib, especially regarding cardiovascular toxicity (Blood; in press). The initial results from Arm C of the SEQUOIA (BGB-3111-304) trial of zanubrutinib in a large cohort of TN CLL/SLL pts with del(17p) were recently presented with a median follow-up of 10 months (Blood 2019;134:851). Presented here is an updated analysis for safety and efficacy in this cohort.
Methods: The SEQUOIA trial (NCT03336333) is an open-label, global, multicenter, phase 3 study that includes a nonrandomized cohort (Arm C) of TN pts with del(17p) CLL/SLL treated with zanubrutinib (160 mg twice daily). Adult pts with CLL/SLL who met International Workshop on CLL (iwCLL) criteria for treatment (Blood 2008;111:5446) were eligible if they were aged ≥65 y or unsuitable for treatment with fludarabine, cyclophosphamide, and rituximab. Use of long-term anticoagulation was permitted. Central verification of del(17p) by fluorescence in situ hybridization with a minimum of 7% aberrant nuclei present was required for entry into Arm C. Response was evaluated by investigator for CLL per modified iwCLL criteria (Blood 2008;112:5259; J Clin Oncol. 2012;30:2820) and for SLL per Lugano criteria (J Clin Oncol. 2014;32:3059).
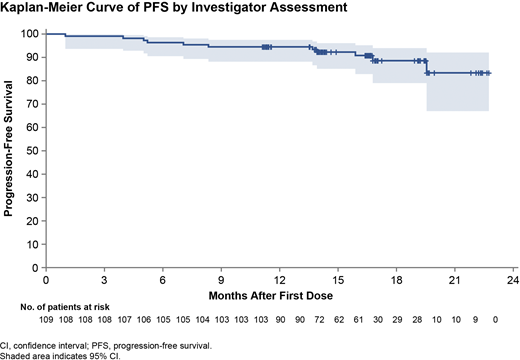
Results: As of 15 Apr 2020 (data cutoff), median follow-up was 18.2 mo (range, 5.0-26.3) for the 109 pts enrolled; 97 pts (89.0%) remained on treatment with zanubrutinib. The best overall response rate (ORR) was 94.5% (3.7% complete response [CR] or CR with incomplete bone marrow recovery, 87.2% partial response [PR], 3.7% PR with lymphocytosis, 4.6% stable disease, 0.9% progressive disease). Five pts (4.6%) met clinical CR criteria but did not undergo bone marrow biopsy. Median progression-free survival (PFS), duration of response (DoR), and overall survival (OS) were not reached. Estimated 18-mo PFS (Figure), 18-mo DoR, and 18-mo OS were 88.6% (95% CI, 79.0-94.0), 84.0% (95% CI, 67.5-92.6), and 95.1% (95% CI, 88.4-97.9), respectively. Investigator-reported transformation occurred in 5 pts (4.6%), of whom 4 had histologic confirmation. Median time to transformation was 13.6 mo (time to transformation for each pt: 3.9, 7.0, 13.6, 13.8, and 15.7 mo). In an exploratory analysis, 37.2% and 26.7% of pts with evaluable karyotypes had at least 3 or 5 distinct karyotypic abnormalities, respectively; no differences in ORR or PFS were observed between pts with or without complex karyotype.
With extended follow-up, adverse events (AEs) reported in ≥10% of treated pts included contusion (20.2%), upper respiratory tract infection (19.3%), neutropenia/neutrophil count decreased (17.4%), diarrhea (16.5%), nausea (14.7%), constipation (13.8%), rash (13.8%), back pain (12.8%), cough (11.9%), arthralgia (11.0%), and fatigue (10.1%). Grade ≥3 AEs occurring in >2% of pts included neutropenia/neutrophil count decreased (12.9%) and pneumonia (3.7%). AEs of interest (pooled terms) included infections (64.2%), bleeding (47.7%; 5.5% grade ≥3 or serious), headache (8.3%), hypertension (8.3%), and myalgia (4.6%). Skin tumors were reported in 9.2%, and non-skin second malignancies were reported in 4.6% of pts. Three pts (2.8%) reported an AE of atrial fibrillation or flutter of which 2 events occurred in the setting of sepsis. Four pts (3.7%) discontinued zanubrutinib due to AEs (including pneumonia, sepsis secondary to Pseudomonas, melanoma, and acute kidney injury [in the context of disease progression]), of which 2 pts have died. Three additional pts died, 2 due to disease progression and 1 from sepsis after progression. No sudden or unknown deaths were reported.
Conclusions: Extended follow-up of zanubrutinib monotherapy in TN CLL/SLL pts with del(17p) showed the durability of responses in this high-risk cohort, with an estimated 18-mo PFS of 88.6% and estimated 18-mo OS of 95.1%. Zanubrutinib was generally well tolerated, with low rates of discontinuation due to AEs. These data support the potential utility of zanubrutinib in the frontline management of pts with high-risk disease.
Brown:TG Therapeutics: Consultancy; Sunesis: Consultancy; MEI Pharma: Consultancy; Nextcea: Consultancy; Novartis: Consultancy; Octapharma: Consultancy; Pfizer: Consultancy; Rigel Pharmaceuticals: Consultancy; Verastem: Consultancy, Research Funding; Kite: Consultancy; Acerta: Consultancy; Genentech: Consultancy; Pharmacyclics: Consultancy; Morphosys: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other; Sun: Research Funding; Loxo: Consultancy, Research Funding; Dynamo Therapeutics: Consultancy; Catapult: Consultancy; BeiGene: Consultancy; Gilead: Consultancy, Research Funding; Invectys: Membership on an entity's Board of Directors or advisory committees, Other: DSMC; Eli Lilly and Company: Consultancy; Astra-Zeneca: Consultancy; Janssen: Honoraria; AbbVie: Consultancy; Juno/Celgene: Consultancy. Robak:Bristol Meyers Squibb: Research Funding; Sandoz: Consultancy, Honoraria; Pfizer: Research Funding; Momenta: Consultancy; UCB: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding; Octapharma: Honoraria; BioGene: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding; Roche: Consultancy, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding; Acerta: Research Funding; GSK: Research Funding; Medical University of Lodz: Current Employment; Morphosys: Research Funding; AbbVie: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding; Takeda: Consultancy; UTX-TGR: Research Funding; Pharmacyclics LLC, an AbbVie Company: Honoraria, Research Funding. Ghia:Novartis: Research Funding; ArQule: Consultancy, Honoraria; Acerta/AstraZeneca: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding; Celgene/Juno: Consultancy, Honoraria; Lilly: Consultancy, Honoraria; MEI: Consultancy, Honoraria; Sunesis: Consultancy, Honoraria, Research Funding; Adaptive, Dynamo: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding; Gilead: Consultancy, Honoraria, Research Funding. Kahl:ADC Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Consultancy; Pharmacyclics LLC: Consultancy; Roche Laboratories Inc: Consultancy; Celgene Corporation: Consultancy; AbbVie: Consultancy; AstraZeneca Pharmaceuticals LP: Consultancy, Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Acerta: Consultancy, Research Funding. Walker:Alfred health: Current Employment; Peninsula Health: Current Employment; Roche: Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company). Janowski:Celgene: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy. Chan:Amgen: Other: TRAVEL, ACCOMODATIONS, EXPENSES (paid by any for-profit health care company); Janssen: Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding, Speakers Bureau; AbbVie: Membership on an entity's Board of Directors or advisory committees; Roche: Other: TRAVEL, ACCOMODATIONS, EXPENSES (paid by any for-profit health care company); Celgene: Other: TRAVEL, ACCOMODATIONS, EXPENSES (paid by any for-profit health care company). Shadman:AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sound Biologics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cellectar: Consultancy, Membership on an entity's Board of Directors or advisory committees; Atara Biotherapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Verastem: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sunesis: Research Funding; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Meyers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; Mustang Bio: Research Funding; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding; Acerta Pharma: Ended employment in the past 24 months; Gilead: Research Funding; MophoSys: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; TG therapeutics: Research Funding. Laurenti:Roche: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Opat:F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZenca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; CSL Behring: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Epizyme: Research Funding. Ciepluch:Copernicus Wojewodzkie centrum Onkologii: Current Employment. Verner:Cilag Pty Ltd: Research Funding; Concord Repatriation General Hospital: Current Employment; Janssen: Research Funding. Šimkovič:University Hospital Hradec Kralove: Current Employment; Acerta Pharma: Consultancy; Gilead Sciences: Consultancy, Other: Travel, Accommodations, Expenses; Janssen-Cilag: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Speakers Bureau; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Speakers Bureau. Österborg:Sanofi: Consultancy; Kancera: Current equity holder in publicly-traded company, Research Funding; BeiGene: Research Funding; Karolinska Univeristy Hospital, Stockholm, Sweden: Current Employment. Trněný:Takeda: Consultancy, Honoraria, Other: TRAVEL, ACCOMODATIONS, EXPENSES (paid by any for-profit health care company); Amgen: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); 1st Faculty of Medicine, Charles University, General Hospital in Prague: Current Employment; MorphoSys: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding; Bristol Meyers Squibb: Consultancy, Honoraria, Other: TRAVEL, ACCOMODATIONS, EXPENSES (paid by any for-profit health care company); Janssen: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); Gilead Sciences: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); Celgene: Consultancy. Tedeschi:BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen spa: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sunesis: Consultancy; Department of Hematology Niguarda Hospital Milano: Current Employment. Blombery:Invivoscribe: Honoraria; Janssen: Honoraria; Amgen: Consultancy; Novartis: Consultancy. Paik:BeiGene: Current Employment, Current equity holder in publicly-traded company. Yin:BeiGene: Current Employment, Current equity holder in publicly-traded company; Arcus Biosciences: Current equity holder in publicly-traded company; Cornell University: Patents & Royalties: A genetically modified mouse model, licensed to pharmaceutical companies. Feng:BeiGene: Current Employment, Current equity holder in publicly-traded company. Ramakrishnan:BeiGene: Current Employment, Current equity holder in publicly-traded company. Huang:BeiGene: Current Employment, Current equity holder in publicly-traded company, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company). Hillmen:F. Hoffmann-La Roche: Honoraria, Research Funding; Astra Zeneca: Honoraria; Gilead: Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding, Speakers Bureau; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding, Speakers Bureau; Pharmacyclics: Research Funding. Tam:BeiGene: Honoraria; AbbVie: Honoraria, Research Funding; Janssen: Honoraria, Research Funding.
Zanubrutinib has not been approved for TN CLL/SLL with del(17p) in the US
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal